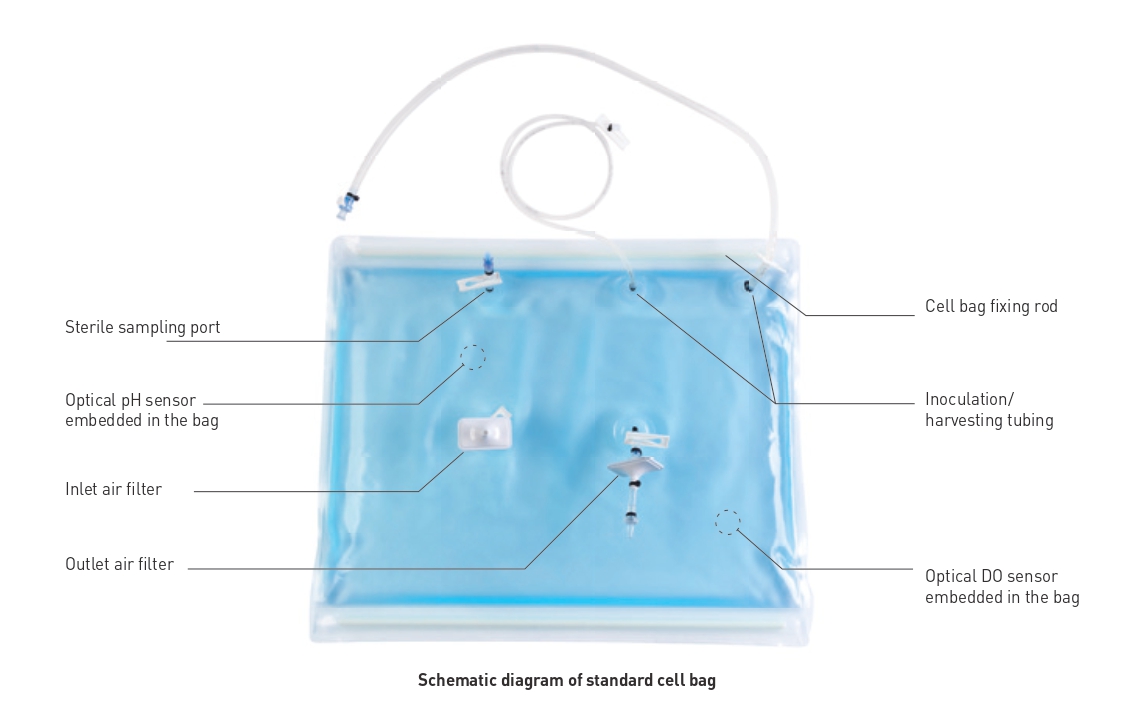
Applications
Suitable for various cell culture conditions, including scientific research, research and development, in-process
seed culture, and new therapies, such as cell therapy. Works with the rocking cell culture systems of GVS or other
major suppliers in the market.
Features
? Easy use: This product is sterile for single use, providing a safe and suitable environment for cell growth, with the features of easy installation and operation
? Good stability: The bags are composed of co-extruded multi-layer films with excellent flexibility and low gas penetration rate, and are suitable for long-term cell culture
? High cell density: The perfusion function enables the high-density cell culture in a faster manner
? Good biosafety: The material liquid contact layer is composed of EVA copolymers, which are biologically inert and can guarantee process safety
? Flexible application conditions: The bags can be used at 10–50 ° C and under operating pressures up to 0.1 bar; the bags are available in various sizes to support culture volumes from 300 mL to 25 L
? Wide selection of bag type: GVS provides cell bags for standard operation, cell therapy, and complex use; optional selections include the basic configuration, for pH & DO, perfusion, and pH & DO & perfusion
? Flexible customization of tubings, connectors, and other units to meet the needs of customers
? Complete validation documents:
? Sterility test
? Bacterial endotoxin test
? Integrity test
? Extractable test
? Chemical compatibility test
? The biocompatibility of gamma-irradiated bags meets the following specifications:
1) ISO 10993-4: In vivo hemolysis test (extraction method)
2) USP87: Cytotoxicity test (extraction method)
3) USP<88> Class VI intramusclar implantation test
4) USP88: Acute intracutaneous test
5) USP88: Acute systemic toxicity test